Gone are the days of carts carrying reams of paper documentation and checklists from station to station on the shop floor. Fortunately, with enterprise content management, document management, collaboration tools, and other digital services, paper is on its way out of the office. But that doesn’t necessarily mean we’re more organized or efficient.
| |
In fact, given the number of electronic documents we receive on every day, it might even be more likely that something important winds up lost, doesn’t get signed when it needs to, or persists as the wrong version.
For issues of quality management, disorganization can easily result in adverse quality events, financial loss, and in some cases unemployment for responsible parties. Enter document management software. It’s been a game changer for small and large organizations, simplifying the document management process with the provision of a standardized and centralized digital warehouse that allows users to easily share and edit documents with the needed security and revision control to comply with a regulated environment.
This article discusses quality management scenarios in which document management is critical, options for quality managers, and adoption rates of software across the enterprise.
Document management software defined
In large organizations, it’s typical for different sites and even departments to have varying types of documents as well as methods for handling them. Without a centralized system to ensure uniformity, there tends to be redundancy in efforts to create and distribute them. Document control software consolidates these efforts, providing a single retrieval and archival resource for controlled documents that is key for effective reporting and easily locating.
Generally delivered over the Internet with role-based security privileges, document management enables communication and collaboration on quality issues. Users can create, edit, link together, and archive documents to act as the enterprise standard. Example documents include standard operating procedures, best practices, training materials, and regulatory content. Documents can also be entered into workflow, facilitating the routing and delivery of certain files to key personnel.
Applying document management to real-world quality scenarios
Today, document management is a typical functionality of enterprise quality management software (EQMS). Because many EQMS functionalities require standardized documents and workflow, document management software is a key enabler of closed-loop quality management—i.e., creating cross-functional quality-data feedback loops to catch problems earlier in the value chain.
Specifically, document control plays a noteworthy role in improving the following processes:
• Change management
• Environment, health, and safety (EH&S)
• Nonconformance/corrective and preventive action (NC/CAPA) management
• Supplier quality management
• Compliance management
• Employee training
• Change management
• Environment, health, and safety (EH&S)
• Nonconformance/corrective and preventive action (NC/CAPA) management
• Supplier quality management
• Compliance management
• Employee training
Where to look for document management options
Although there is a strong and existing market for cloud storage and file synchronization software that’s leveraged by consumers and businesses alike, there are a number of reasons why a quality organization requires more than what DropBox, Google Docs, or similar services have to offer. Document control software is often prebuilt to have the following capabilities:
• Security/role-based access
• Version control
• Document workflow capabilities (routing, delivery, approval, escalation, etc.)
• Security/role-based access
• Version control
• Document workflow capabilities (routing, delivery, approval, escalation, etc.)
As mentioned previously, because the solution is an enabler of closed-loop quality, the functionalities are often built with the context of manufacturing and quality processes in mind for more interoperability.
Additionally, independent software vendors have used different technologies to develop solutions, including technology from IBM, Oracle, and more recently, Microsoft SharePoint. Quality organizations looking to invest in a document management solution may consider the following providers that were included in the 2012 EQMS Solution Selection Guide:
• Intelex
• EtQ
• MetricStream
• Qumas
• IBS
• IQS
• CEBOS
• Sword Achiever
• Intelex
• EtQ
• MetricStream
• Qumas
• IBS
• IQS
• CEBOS
• Sword Achiever
There are also a few EQMS vendors not in the guide that offer document management and may be worth a look, including: NextDocs, MasterControl, and Pilgrim Software. Finally, in some cases a company may want to use a more robust stand-alone content management system that integrates with EQMS. Commonly used providers include: EMC Documentum, Oracle, IBM, Microsoft, and Hyland Software.
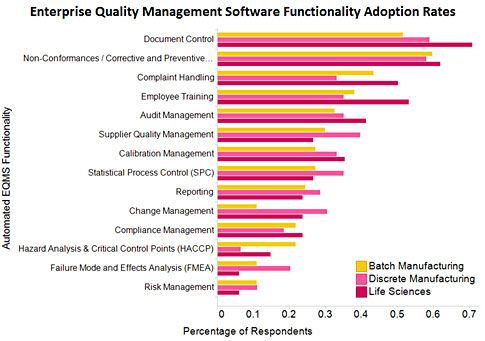
Adoption of enterprise document management software in EQMS
The LNS Research 2012–2013 Quality Management Survey asked executives and quality leaders about their 2012 performance in key metrics and about their technology adoption. The chart below shows the adoption rates of specific EQMS functionalities.
Source:
Document Management Options for Quality Managers | Quality Digest

Nenhum comentário:
Postar um comentário